Part:BBa_K660100
Ranaspumin-2 (Surfactant Protein)
Ranaspumin 2 is one of six proteins used by the tungara frog (Engystomops pustulosus, found in Central and South America) to form protein foam nests in which it deposits its eggs. Protein foams are relatively rare in biology, and in this case offer a biocompatible solution to incubation and protection from mechanical destruction, as well as anti-microbial properties.

Protein foam nest from Trinidad 1 |

A protein foam nest containing newly hatched frogspawn 2 |

Pustulosus constructing a foam nest in the wild 2 |
Structure
Coded for by the gene rsn-2, Ranaspumin2 (Rsn2) is a monomeric surfactant protein, with an atomic mass of 11kDa. It has a molecular weight of 13415.2 and is comprised of 117 amino acids. In aqueous solution it adopts a tightly folded globular shape, which is uncharacteristic of surfactant proteins. In this 3D arrangement Rsn2 consists of a single helix over a four-stranded sheet, as can be seen in the image below.
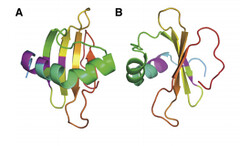
Image 1: Ribbons structure of Ranaspumin2 A: Frontal view B: 90 degree-rotation (Mackenzie, 2009)
This structure is highly unusual for a protein with surfactant properties, and appears to convey no significant amphiphilicity. It is therefore believed that Rsn2 undergoes a significant conformational change when at the air-water interface, in which it will expose hydrophobic groups to the air and hydrophilic groups to the water beneath. (Mackenzie, 2009)
Illegal Restriction Sites
There are no illegal restriction sites present in our Ranaspumin-2 biobrick.Function
The protein undergoes a conformational refolding whilst in solution, exhibiting significant surfactant properties at the air-water interface. This property makes it potentially useful when working with biofilms that form at the same phase interface, as it is thought that it may assist in the destruction of the biofilm.
ContributionGroup: Austin UTexas
Summary: According to Mackenzie et al., Rsn-2 in solution adopts a compact globular fold characteristic of the cystatin family, comprising a single helix over a four-stranded sheet, in a motif not previously associated with surfactant activity. However, when Rsn-2 comes into contact with a surface of water, it is able to decrease surface tension even at low concentrations. Rsn-2 will readily adsorb to both air-water and air-oil interfaces. According to Brandani et al., this function is facilitated by a conformation change that opens the enzyme, exposing its interior to the interface. Rsn-2’s hydrophobic N-terminus mediates adsorption of the protein to the air-water interface. Mutations in either the hydrophobic N-terminus or the hydrophilic C-terminus affect the protein’s function as a surfactant. This decrease in surface tension can be used to destroy biofilms accumulated at oil-water interfaces.
References
Mackenzie et al., 2009. Ranaspumin-2: structure and function of a surfactant protein from the foam nests of a tropical frog. Biophysical Journal, 96, pp. 4984-4992
Kennedy, M.W., Proteins of Frog Foam Nests. Date Accessed: 20/09/2011
Cooper, A., Biofoams and natural protein surfactants Date Accessed: 24/08/1991
Brandani, G., Vance, S., Schor, M., Cooper, A., Kennedy, M., & Smith, B. et al. (2017). Adsorption of the natural protein surfactant Rsn-2 onto liquid interfaces. Physical Chemistry Chemical Physics, 19(12), 8584-8594. doi: 10.1039/c6cp07261e
Sequence and Features
- 10COMPATIBLE WITH RFC[10]
- 12COMPATIBLE WITH RFC[12]
- 21COMPATIBLE WITH RFC[21]
- 23COMPATIBLE WITH RFC[23]
- 25INCOMPATIBLE WITH RFC[25]Illegal AgeI site found at 66
- 1000COMPATIBLE WITH RFC[1000]
| None |
